Answer: D) a positive ΔH, absorbs heat from the surroundings, and feels cold to the touch.
Step-by-step explanation:
The enthalpy of reaction, ΔH, is the difference between the enthalpies of the products and the enthalpies of the reactants:
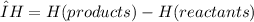
The enthalpy of reaction can be positive or negative, depending on the process. For an endothermic process (heat absorbed by the system from the surroundings), ΔH is positive (that is, ΔH is greater than 0). Since the system is absorbing heat, it feels cold to the touch.
For an exothermic process (heat released by the system to the surroundings), ΔH is negative (that is, ΔH is smaller than 0). Since the system is releasing heat, it feels warm to the touch.