Answer: The temperature in degrees Celsius is 51.21ºC.
Step-by-step explanation:
An ideal gas is a hypothetical gas whose pressure-volume-temperature
behavior can be completely accounted for by the ideal gas equation:
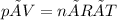
We want to know the temperature of the sample, which will be in Kelvin, so we rearrange the equation to give:
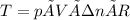
Knowing that:
p= 2.69 atm
n= 6.50 mol
V= 64. 27 L
and R, the gas constant is 0.082 L× atm ÷ mol×K
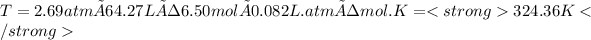
Now, we need to convert Kelvins to degrees Celsius. We use the following equation:
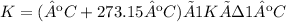
K= 324.36 K - 273.15ºC = 51.21 ºC
Therefore, the temperature of the sample is 51.21 degrees Celsius.