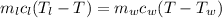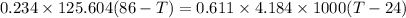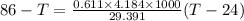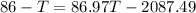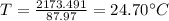Answer:
Step-by-step explanation:
Given
mass of lead piece
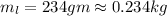
mass of water in calorimeter
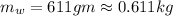
Initial temperature of water
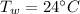
Initial temperature of lead piece
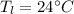
we know heat capacity of lead and water are
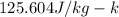 and
and
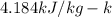 respectively
respectively
Let us take
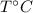 be the final temperature of the system
be the final temperature of the system
Conserving energy
heat lost by lead=heat gained by water