Answer:
t = 25.10 sec
Step-by-step explanation:
we know that Avrami equation
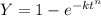
here Y is percentage of completion of reaction = 50%
t is duration of reaction = 146 sec
so,
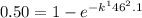
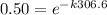
taking natural log on both side
ln(0.5) = -k(306.6)
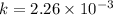
for 86 % completion
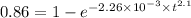
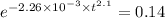
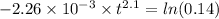
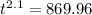
t = 25.10 sec