Answer:
Final temperature will be T = 67.68°C
Step-by-step explanation:
The heat evolved by the copper tubing will be absrobed by both water and the vessel used.
The heat evolved by the copper tubing will be:
Heat =
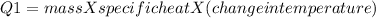
Mass = 670 g
Specific heat = 0.387 J/g · K
Change in temperature = Initial - Final
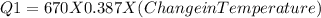
The heat absorbed by water will be
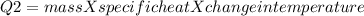
mass = 52.5
Specific heat = 4.184 J/g · K
the heat absorbed by vessel will be:
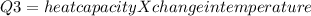
Heat capacity = 10J/K
Final temperature of all the three will be same (say T)
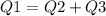
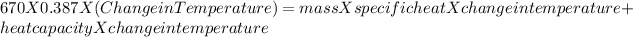
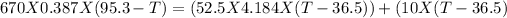
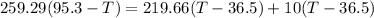
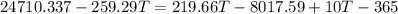
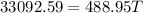
T = 67.68°C