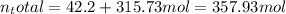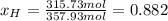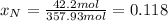Answer:
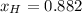
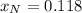
Step-by-step explanation:
In this reactor, oleic and linoleic acid react with hydrogen to form stearic acid. This reactions can be represented by:
Oleic:
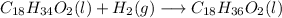
Linoleic:
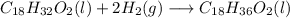
Having this reactions in mind, the first thing is to determine the moles of hydrogen required:
Base of caculation: 1 mol of sunflower oil
For oleic acid:
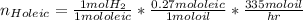
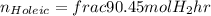
For linoleic acid:
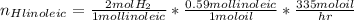
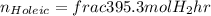
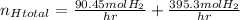
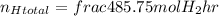
Applying the excess:
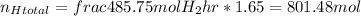
Nitrogen:
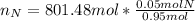
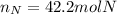
After the reactions:
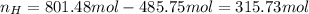
and the nitrogen is inert.
Purge stream: