Answer:
mass percent of chlorine is 56.5%
Step-by-step explanation:
Given data:
Volume V = 581 mL = 0.581 L
Pressure P = 752 mm of Hg = 752/760 = 0.9894 atm
Temperature T = 298 K
Molar gas constant R = 0.08206 atm.L/mol.K
from Ideal gas equation we have following relation
PV = nRT solving for n
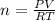

n = 0.0235 mole
mas of
 = moles × molar mass of
= moles × molar mass of


Mass percent of chlorine

