Answer:
change in entropy is 1.44 kJ/ K
Step-by-step explanation:
from steam tables
At 150 kPa
specific volume
Vf = 0.001053 m^3/kg
vg = 1.1594 m^3/kg
specific entropy values are
Sf = 1.4337 kJ/kg K
Sfg = 5.789 kJ/kg
initial specific volume is calculated as
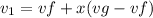
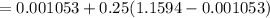
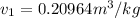
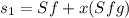
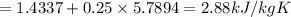
FROM STEAM Table
at 200 kPa
specific volume
Vf = 0.001061 m^3/kg
vg = 0.88578 m^3/kg
specific entropy values are
Sf = 1.5302 kJ/kg K
Sfg = 5.5698 kJ/kg
constant volume so
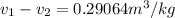
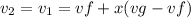
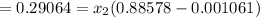
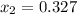
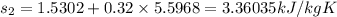
Change in entropy
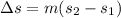
=3( 3.36035 - 2.88) = 1.44 kJ/kg