Answer: 18.97 L
Step-by-step explanation:
This can be solved by the Ideal Gas equation:
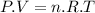
Where:
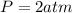 is the pressure of the gas
is the pressure of the gas
 is the volume of the gas
is the volume of the gas
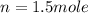 the number of moles of gas
the number of moles of gas
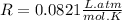 is the gas constant
is the gas constant
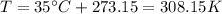 is the absolute temperature of the gas in Kelvin
is the absolute temperature of the gas in Kelvin
Finding
 :
:
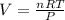
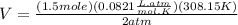
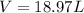
Therefore:
The volume occupied by 1.5 mole of gas at 35°C and 2.0 atmosphere of pressure is 18.97 liters