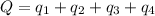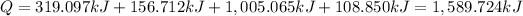Answer:
Heat required to convert 2.500 kg of solid SO₂ at the melting point to a gas at 60˚C is 1,589.724 kilo joules.
Step-by-step explanation:
Heat required to melt sulfur dioxide solid -73˚C =
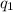
Latent heat melting of sulfur dioxide =
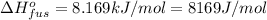
Mass of sulfur dioxide solid , m= 2.500 kg = 2500 g
Moles of sulfur dioxide solid ,n=
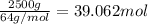
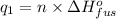
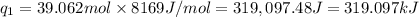
Heat required to raise the temperature of liquid sulfur dioxide from -73˚C to -10˚C =
-10˚C = 263.15 K, -73˚C = 200.15 K
Mass of sulfur dioxide liquid, m= 2.500 kg = 2500 g
Specific heat of liquid sulfur dioxide =
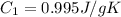
Change in temperature =
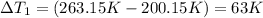
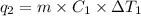
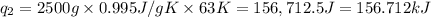
Heat required to vaporize sulfur dioxide liquid at -10˚C=
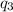
Latent heat melting of sulfur dioxide =
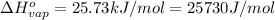
Mass of sulfur dioxide liquid, m= 2.500 kg = 2500 g
Moles of sulfur dioxide solid ,n=
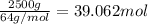
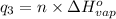
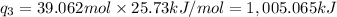
Heat required to raise the temperature of liquid sulfur dioxide from -10˚C to 60˚C =
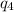
-10˚C = 263.15 K, 60˚C = 333.15 K
Mass of sulfur dioxide liquid, m= 2.500 kg = 2500 g
Specific heat of gas sulfur dioxide =
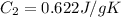
Change in temperature =
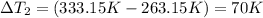
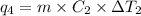
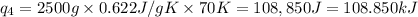
Heat required to convert 2.500 kg of solid SO₂ at the melting point to a gas at 60˚C = Q