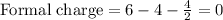Answer:
A.) 0
Step-by-step explanation:
Dinitrogen oxygen (
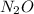 )
)
Valence electrons of oxygen = 6
Valence electron of nitrogen = 5
The total number of the valence electrons = 2(5) + 6 = 16
The Lewis structure is drawn in such a way that the octet of each atom and duet for the hydrogen in the molecule is complete. So, The Lewis structure is shown in image below.
Formula for formal charge :
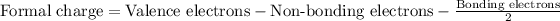
Formal charge for oxygen: