Answer:
Molarity of the unknown acid is 0,76M
Step-by-step explanation:
In an acid base reaction:
HA + NaOH → NaA + H₂O
The moles of NaOH that you spend in the titration will be the same of the HA. Thus:
Volume spend ×molarity=moles of NaOH
(0,04117L-0,00163L)×0,288M = 0,0114 moles NaOH ≡ moles of HA
The sample of the unknown acid has a volume of 15,0mL=0,0150L. Thus, molarity of the unknown acid is:
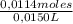 = 0,76M
= 0,76M
I hope it helps!