Answer:
Density of carbon tetrachloride = 12.8 g/mL
Step-by-step explanation:
Given :
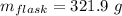
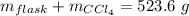
Mass of carbon tetrachloride: -
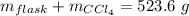
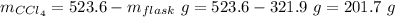
Mass of carbon tetrachloride = 201.7 g
Given, Volume = 15.7 mL
Considering the expression for density as:
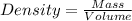
So,
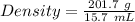
Density of carbon tetrachloride = 12.8 g/mL