Answer:
The balanced reaction is:-
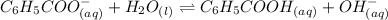
 expression is:-
expression is:-
![K_(b)=\frac {\left [ C_6H_5COOH \right ]\left [ {OH}^- \right ]}{[C_6H_5COO^-]}](https://img.qammunity.org/2020/formulas/chemistry/college/ak5d9selwahpogf5syvc6fjooaam6snt3h.png)
Step-by-step explanation:
Benzoate ion is the conjugate base of the benzoic acid. It is a Bronsted-Lowry base and the dissociation of benzoate ion can be shown as:-
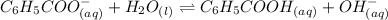
The expression for dissociation constant of benzoate ion is:
![K_(b)=\frac {\left [ C_6H_5COOH \right ]\left [ {OH}^- \right ]}{[C_6H_5COO^-]}](https://img.qammunity.org/2020/formulas/chemistry/college/ak5d9selwahpogf5syvc6fjooaam6snt3h.png)