Answer:
D. The new rms speed is 1.732 times the original rms speed.
Step-by-step explanation:
The expression for the root mean square speed is:
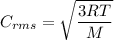
R is Gas constant having value = 8.314 J / K mol
M is the molar mass of gas
T is the absolute temperature
As seen from the formula, root mean square speed is directly proportional to the square root of the absolute temperature.
So,
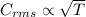
Given, absolute temperature of a gas is tripled, so, the new rms speed will be √3 (1.732) of the original.
Hence, the correct option is:- D. The new rms speed is 1.732 times the original rms speed.