Answer:
All the options beside A are correct.
Step-by-step explanation:
Oxidation reaction is defined as the reaction in which an atom looses its electrons. Here, oxidation state of the atom increases.
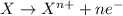
Reduction reaction is defined as the reaction in which an atom gains electrons. Here, the oxidation state of the atom decreases.
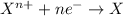
So, when metal gets oxidized by the other substance , there is direct contact of metal and oxidizer.
During corrosion of metal ,there is a loss of electrons from the metal atom which increases its oxidation state and other oxidizer itself get reduced.