Answer:
-24.76 kJ/mol
Step-by-step explanation:
given,
mass of solid magnesium burned = 0.1375 g
the temperature increases by(ΔT) 1.126°C
heat capacity of of bomb calorimeter (C_{cal})= 3024 J/°C
heat absorbed by the calorimeter

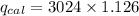
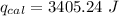
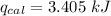
heat released by the reaction
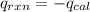
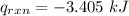
energy density will be equal to heat released by the reaction divided by the mass of magnesium
Energy density =
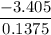
Energy density = -24.76 kJ/mol
heat given off by burning magnesium is equal to -24.76 kJ/mol