Answer:
The work for the given process is (- 2263.4 J)
Step-by-step explanation:
Given: Mass of nitrogen gas (N₂): w = 92 g, external pressure: P = 1 atm, Initial temperature: T₁ = 200 K, Final temperature: T₂ = 200 + 83 K = 283 K
Molar mass of N₂ gas: m = 28 g, Gas constant: R = 8.314 J.K⁻¹.mol⁻¹
The number of moles of N₂ gas = w ÷ m = 92 g ÷ 28 g/mol = 3.28 mole
To find the initial volume (V₁) and final volume (V₂), we use the ideal gas equation:
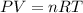
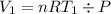
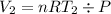
So the Work:
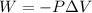
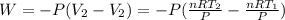
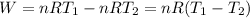
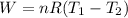
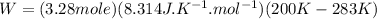
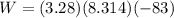
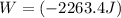
Therefore, the work for the given process: W = (- 2263.4 J)