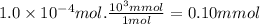Answer:
0.10 mmol
Step-by-step explanation:
Initially there is only HCl in the beaker. We can calculate the moles of HCl using the following expression:
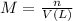
where,
M is the molarity of the solution
n is the moles of the solute
V(L) is the volume of the solution expressed in litres
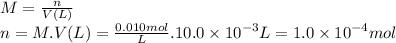
The milimoles of HCl are: