Answer:
4.24 L
Step-by-step explanation:
We are given;
- Initial Volume, V1 is 3.10 L
- Initial temperature, T1 is 13.80°C
But, K = °C + 273.15
- Thus, Initial temperature, T1 is 286.95 K
- Initial pressure, P1 is 1.30 atm
- New temperature, T2 is 25°C or 298.15 K
- New pressure, P2 is 0.988 atm
We are required to calculate the new volume;
- We are going to use the combined gas law;
- According to the combined gas law;
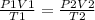
Rearranging the formula;
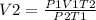
Therefore;
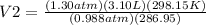
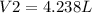
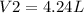
Therefore, the new volume of the gas sample is 4.24 L