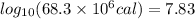Answer: a)
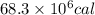
b)
Step-by-step explanation:
Heat is defined as a spontaneous flow of energy from one object to another. It is measured in Joules, calories, kilo Joules etc.
These units of energy are inter convertible.
We are given:
a) Energy absorbed by limestone =
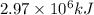
Converting this unit of temperature into
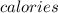 by using conversion factor:
by using conversion factor:
1 kJ = 239.006 calories
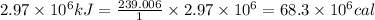
Thus the energy in calories is
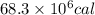
b) The value of log base 10 of
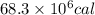 is:
is: