Answer:
mass = 0.44 Kg
Step-by-step explanation:
given,
Calories contain in the strawberry yogurt = 255 calories
1 Calorie = 4186 J
latent heat of vaporization = 2.42 x 10⁶ J/Kg
mass of perspiration = ?
Energy = 255 calories
Energy = 255 x 4186
= 1067430 J
Energy = mass x latent heat of vaporization
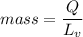
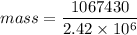
mass = 0.44 Kg