Answer:
The false statement regarding an exothermic reaction is: A. the products have a higher enthalpy than reactants
Step-by-step explanation:
An exothermic reaction is a type of chemical reaction that involves the release of energy from the system to the surroundings. Thus increasing the temperature of the surroundings.
In this reaction, the enthalpy or energy of the reactants is greater than the enthalpy or energy of the products. (
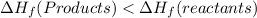 )
)
As the enthalpy change of a reaction:
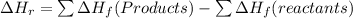
Therefore, the enthalpy change for an exothermic reaction is negative (
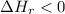 )
)