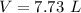Answer:
Step-by-step explanation:
Given:
Initial temperature of water,
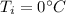
final temperature of water,
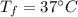
energy spent in one hour of walk,
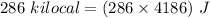
volumetric capacity of stomach,
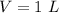
Now, let m be the mass of water at zero degree Celsius to be drank to spend 286 kilo-calories of energy.
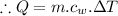 .....................................(1)
.....................................(1)
where:
m = mass of water
Q = heat energy
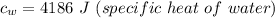
 = temperature difference
= temperature difference
Putting values in the eq. (1):
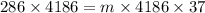
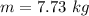
Since water has a density of 1 kilogram per liter, therefore the volume of water will be: