Answer:
A)
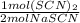
B) 0.025 mol (SCN)₂
C) 2 mol (SCN)₂
D)
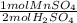
E) 3.5504 mol H₂SO₄
Step-by-step explanation:
2NaSCN +2H₂SO₄ + MnO₂ → (SCN)₂ + 2H₂O + MnSO₄ + Na₂SO₄
A) A conversion factor could be
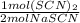 , as it has the units that we want to convert to in the numerator, and the units that we want to convert from in the denominator.
, as it has the units that we want to convert to in the numerator, and the units that we want to convert from in the denominator.
B) 0.05 mol NaSCN *
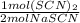 = 0.025 mol (SCN)₂
= 0.025 mol (SCN)₂
C) With 4 moles of NaSCN and 3 moles of MnSO₄, the reactant is NaSCN so we use that value to calculate the moles of product formed:
4 mol NaSCN *
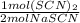 = 2 mol (SCN)₂
= 2 mol (SCN)₂
D)
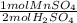
E) 1.7752 mol MnSO₄ *
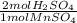 = 3.5504 mol H₂SO₄
= 3.5504 mol H₂SO₄