Answer:
72.57 grams
Step-by-step explanation:
The mass percentage of gold in the ore is 0.19 %
The mass of ore used = 23 kg
The mass of gold in the ore =
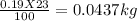
moles of gold in the given mass of ore =
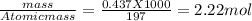
As per given equation four moles of Au is giving four moles of complex compound on reacting with NaCN
Then in second reaction two moles of the complex is reacting with one mole of Zinc
Thus two moles of gold are reacting with one mole of Zinc
The moles of Zinc needed = 0.5 X 2.22 mol = 1.11 moles
The mass of Zinc needed = moles X atomic mass =1.11 X 65.38 = 72.57 grams