Answer:
Molarity = 1.53 M
Step-by-step explanation:
At STP,
Pressure = 1 atm
Temperature = 273.15 K
Given, Volume = 27.0 L
Using ideal gas equation as:

where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L.atm/K.mol
Applying the equation as:
1 atm × 27.0 L = n × 0.0821 L.atm/K.mol × 273.15 K
⇒n = 1.20 moles
Given that volume = 785 mL
Also,
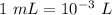
So, Volume = 785 / 1000 L = 0.785 L
Considering:
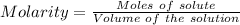
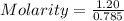
Molarity = 1.53 M