Answer:
C. Phosphorus (P)
Step-by-step explanation:
Given;
Electron configuration
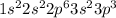
Firstly we have to find out the number of shell of given element. For this we have to add number of electron of each shell.
Here counting goes to 3 that means element have three shells. Now we find out number of electrons in each shell.
First shell of an element is K and it has only one sub shell that is 1s. In this element 1s contains only 2 electrons.
For 2nd shell that is L has two sub shell 2s and 2p, for this element 2s contains 2 electrons and 2p contains 6 electrons. That means total number of electrons for L shell is 8.
Now for 3rd shell that is M has two sub shell 3s and 3p, for this element 3s contains 2 electrons and 3p contains 3 electrons. That means total number of electrons for M shell is 5.
Now total number of electrons for the element is the sum of number of electrons in each shell.
Total number of electrons = 2+8+5=15
And we know the number of electrons of any neutral element is equal to the number of protons and number of protons gives us atomic number of the element.
So the atomic number of given element is 15 that is of Phosphorus.