Answer:
427.392 kJ
Step-by-step explanation:
m = Mass of gas = 4.5 kg
Initial temperature = 200 K
Final temperature = 360 K
R = Mass specific gas constant = 296.8 J/kgK
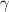 = Specific heat ratio = 1.5
= Specific heat ratio = 1.5
Work done for a polytropic process is given by
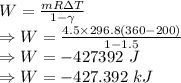
The work input during the process is -427.392 kJ