Answer:
A solution that is 0.100 M (i.e. 33,12 g of lead (II) nitrate in 1.00 L of water) yields the desired total dissolved ions concentration.
Step-by-step explanation:
The molecular formula of lead (II) nitrate is Pb(NO
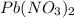 and its molecular mass is 331,2 g/mol.
and its molecular mass is 331,2 g/mol.
In disolution, the equilibrium will look like this:
Pb(NO
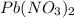 ->
->
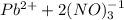
The equation above means that, one mol of lead (II) nitrate dissolved in 1L will yield one mol of Pb ions and 2 moles of NO3 ions, i.e. 3 moles total.
If we dissolve 0.100 moles of lead (II) nitrate in 1.00 L of water, the stoichiometry of the disolution states that in turn, it will yield 0.100 of Pb ions and 0.200 moles of NO3 ions, i.e. 0.300 M in total dissolved ions.
331,2 g/mol * 0.100 mol/L * 1 L = 33,12 grams of the compound are required.