Answer:
93,32 % (w/w)
Step-by-step explanation:
The Ca²⁺ of CaCO₃ was completely precipitate to CaC₂O₄ that results in H₂C₂O₄. The titration of this one is:
3H₂C₂O₄ + 2H⁺ + MnO₄⁻ → Mn²⁺ + 4H₂O + 6CO₂
As you required 35,62mL of 0,1092M MnO₄⁻, the moles of H₂C₂O₄ are:
0,03562L×
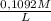 =
=
3,890x10⁻³mol of MnO₄⁻×
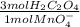 = 0,01167 mol of H₂C₂O₄
= 0,01167 mol of H₂C₂O₄
The number of moles of CaCO₃ are the same number of moles of H₂C₂O₄ because every Ca²⁺ was converted in CaC₂O₄ that was converted in H₂C₂O₄. That means: 0,01167 mol of CaCO₃
0,01167 mol of CaCO₃ are:
0,01167 mol of CaCO₃×
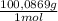 = 1,168 g of CaCO₃
= 1,168 g of CaCO₃
As the mass of the initial mixture is 1,2516 g, the percentage by weight of CaCO₃ is:
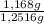 ×100 = 93,32 % (w/w)
×100 = 93,32 % (w/w)
I hope it helps!