Answer:
M=28.88 gm/mol
Step-by-step explanation:
Given that
T= 95 K
P= 1.6 atm
V= 4.87 L
m = 28.6 g
R=0.08206L atm .mol .K
We know that gas equation for ideal gas
P V = n R T
P=Pressure , V=Volume ,n=Moles,T= Temperature ,R=gas constant
Now by putting the values
P V = n R T
1.6 x 4.87 = n x 0.08206 x 95
n=0.99 moles
We know that number of moles given as
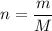
M=Molar mass
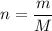
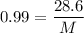
M=28.88 gm/mol