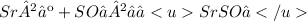Answer:
1. Strong electrolytes: HCl and NaOH.
Weak electrolytes: CH₃COOH and NH₃.
Nonelectrolytes: (NH₂)₂CO (urea) and CH₃OH (methanol).
2. C)
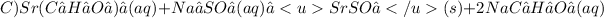
3. The spectator ions are Na⁺ and C₂H₃O₂⁻.
4. Written in explanation section.
Step-by-step explanation:
- An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity.
A characteristic of strong electrolytes is that the solute is assumed to be 100 percent dissociated into ions in solution, therefore they are great conductors of electricity. Eg: HCl and NaOH.
On the other hand, weak electrolytes are not completely dissociated in solution, therefore they are poor conductors of electricity. Eg: CH₃COOH and NH₃.
(By dissociation we mean the breaking up of the compound into cations and anions).
A nonelectrolyte does not conduct electricity when dissolved in water. Eg: (NH₂)₂CO (urea) and CH₃OH (methanol).
2. The products formed on each reaction are (always remember to balance the equations):
A)
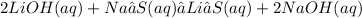
B)
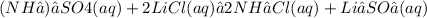
C)
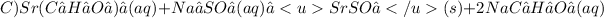
D)
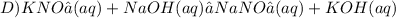
The reaction C will produce SrSO₄, a white color precipitate.
3. When ionic compounds dissolve in water, they break apart into their component cations and anions. To be more realistic, the equations should show the dissociation of dissolved ionic compounds into ions. This is called a ionic equation which shows dissolved species as free ions. To see whether a precipitate might form from this solution, we first combine the cation and anion from different compounds, and refer to the solubility rules. The spectator ions are ions that are not involved in the overall reaction.
Therefore, for the equation chosen above:
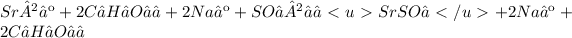
Because spectator ions appear on both sides of an equation, they can be eliminated from the ionic equation.
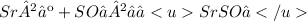
Finally, we end up with the net ionic equation, which shows only the species that actually take part in the reaction.
In this reaction, the spectator ions are Na⁺ and C₂H₃O₂⁻.
4. Molecular equations:
A)
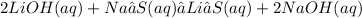
B)
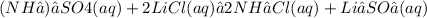
C)
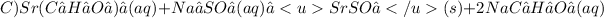
D)
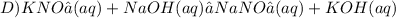
Complete ionic equations:
A)
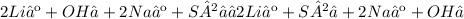
B)
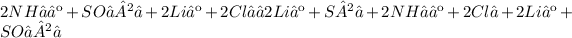
C)
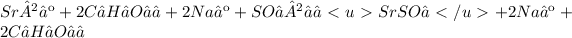
D)
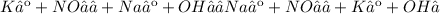
If all products are aqueous, a net ionic equation cannot be written because all ions are canceled out as spectator ions. Therefore, no precipitation reaction occurs. The only net equation can be written for reaction C):
C)