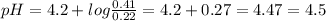Answer:
The pH will be 4.5
Step-by-step explanation:
The mixture of benzoic acid (weak acid) and its salt will make a buffer.
The pH of buffer solution can be calculated using Henderson Hassalbalch's equation, which is
![pH=pKa+log([salt])/([acid])](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xlklcv7b1496sz2ozqct518piznfdmytqu.png)
pKa = -logKa
pKa = -log(
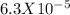 )
)
pKa = 4.2