Answer:
The correct option is: a. The internal energy depends upon its temperature.
Step-by-step explanation:
Ideal gas is a hypothetical gas that obeys the ideal gas law. The equation for the ideal gas law:
P·V=n·R·T
Here, V- volume of gas, P - total pressure of gas, n- total mass or number of moles of gas, T - absolute temperature of gas and R- the gas constant
Also, according to the Joule's second law, the internal energy (U) of the given amount of ideal gas depends on the absolute temperature (T) of the gas only, by the equation:
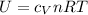
Here,
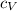 is the specific heat capacity at constant volume
is the specific heat capacity at constant volume