Answer: 37.8 grams
Explanation:-
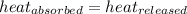
As we know that
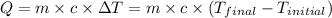
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2020/formulas/chemistry/college/bm2kxludvecqgwsul6e5upktie71evfnq2.png) .................(1)
.................(1)
where,
q = heat absorbed or released
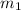 = mass of iron rod = 32.4 g
= mass of iron rod = 32.4 g
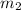 = mass of water = ?
= mass of water = ?
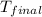 = final temperature =
= final temperature =
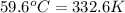
 = temperature of iron rod =
= temperature of iron rod =
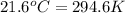
 = temperature of water =
= temperature of water =
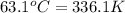
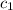 = specific heat of iron rod =
= specific heat of iron rod =
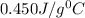
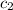 = specific heat of water=
= specific heat of water=
Now put all the given values in equation (1), we get
![32.4* 0.450* (332.6-294.6)=-[m_2* 4.18* (332.6-336.1)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/dokazs3tcdc0w7t3gg0t9edcov8sauofoc.png)
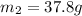
Therefore, the mass of the water is 37.8 grams