Answer:
The final volume of air after compression: V₂ = 4477.63 L = 158.12 cf
Step-by-step explanation:
Given: Initial gauge pressure of the gas = 0 psi
Initial absolute pressure of the gas: P₁ = gauge pressure + atmospheric pressure = 0 psi + 12.20 psi = 12.20 psi
Initial Temperature = 70°F
⇒ T₁ = (70°F − 32) × 5/9 + 273.15 = 294.26 K
Initial volume of the gas: V₁ = 1200 cf = 1200 × 28.317 = 33980.4 L (∵ 1 cf ≈ 28.317 L)
Final gauge pressure of the gas = 90 psi
Final absolute pressure of the gas: P₂ = gauge pressure + atmospheric pressure = 90 psi + 12.20 psi = 102.2 psi
Final Temperature: T₂ = 125°F
⇒ T₂ = (125°F − 32) × 5/9 + 273.15 = 324.82 K
Final volume of the gas: V₂ = ? L
According to the Combined gas law:
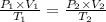
→
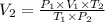
→
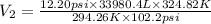
→
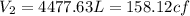
Therefore, the final volume of air after compression: V₂ = 4477.63 L = 158.12 cf