Answer:
Solubility of X in water at 17°C is > 0,05 kg/L
Step-by-step explanation:
When the solution is at 17°C the solution remains clear. That means that every X molecule is dissolved in water. The student obtained 0,15 kg of X in 3,00L of water. That means that under this conditions the concentration is:
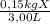 = 0,05kg/L
= 0,05kg/L
The solubility is defined as the concentration of a saturated solution. A saturated solution is a solution in which the solute (X) is in the maximum possible concentration. That means that solubility of X in water at 17°C is > 0,05 kg/L because X was completely dissolved in water and there is not enough data to obtain the exact solubility.
You need to add more X, heat the solution and then, cool it at 17°C obtaining a precipitate. The concentration of X substracting the mass of X that precipitate is the solubility of X in water.
I hope it helps!