Answer:
There was 62.23% change in radius as the ion formed.
Explanation:
Given
Radius of Aluminium
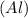 atom = 143 pm
atom = 143 pm
Radius of Aluminium
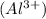 atom = 54 pm
atom = 54 pm
Change is the radius = Radius of Aluminium
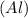 atom - Radius of Aluminium
atom - Radius of Aluminium
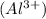 atom = 143 -54 = 89
atom = 143 -54 = 89
Now to find % Change is the radius we will divide Change is the radius by Radius of Aluminium
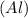 atom and multiply by 100 we get
atom and multiply by 100 we get
% Change is the radius =
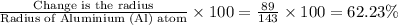
Hence there was 62.23% change in radius as the ion formed.