Answer:
0.174 M
Step-by-step explanation:
The same can be solve by using Nernst's equation
The Nernst's equation is:
![Ecell=E^(0)_(cell)-(0.0592)/(n)log([anodic])/([cathodic])](https://img.qammunity.org/2020/formulas/chemistry/college/8g9d2b8xrrgzhh74bstjmt89qn5bvdimos.png)
For silver cell
n= 1
As both the compartments have silver nitrate solution,t the standard emf of cell will be zero.
Given
Ecell = 0.045
[Anodic compartment]= 1 M
Putting values
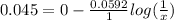
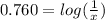
Taking antilog and solving
[AgNO3]=0.174 M