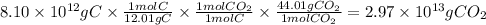Answer:
2.97 × 10¹³ g
Step-by-step explanation:
First, we have to calculate the biomass the is burned. We can establish the following relations:
- 2.47 acre = 10,000 m²
- 10 kg of C occupy an area of 1 m²
- 50% of the biomass is burned
The biomass burned in the site of 400,000 acre is:
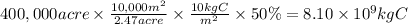
Let's consider the combustion of carbon.
C(s) + O₂(g) ⇒ CO₂(g)
We can establish the following relations:
- The molar mass of C is 12.01 g/mol
- 1 mole of C produces 1 mole of CO₂
- The molar mass of CO₂ is 44.01 g/mol
The mass of produced is CO₂: