Answer:
0,07448M of phosphate buffer
Step-by-step explanation:
sodium monohydrogenphosphate (Na₂HP) and sodium dihydrogenphosphate (NaH₂P) react with HCl thus:
Na₂HP + HCl ⇄ NaH₂P + NaCl (1)
NaH₂P + HCl ⇄ H₃P + NaCl (2)
The first endpoint is due the reaction (1), When all phosphate buffer is as NaH₂P form, begins the second reaction. That means that the second endpoint is due the total concentration of phosphate that is obtained thus:
0,01862L of HCl×
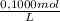 = 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.
= 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.
The concentration is:
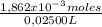 = 0,07448M of phosphate buffer
= 0,07448M of phosphate buffer
I hope it helps!