Answer:
4) Each cytochrome has an iron‑containing heme group that accepts electrons and then donates the electrons to a more electronegative substance.
Step-by-step explanation:
The cytochromes are proteins that contain heme prosthetic groups. Cytochromes undergo oxidation and reduction through loss or gain of a single electron by the iron atom in the heme of the cytochrome:
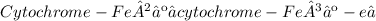
The reduced form of ubiquinone (QH₂), an extraordinarily mobile transporter, transfers electrons to cytochrome reductase, a complex that contains cytochromes b and c₁, and a Fe-S center. This second complex reduces cytochrome c, a water-soluble membrane peripheral protein. Cytochrome c, like ubiquinone (Q), is a mobile electron transporter, which is transferred to cytochrome oxidase. This third complex contains the cytochromes a, a₃ and two copper ions. Heme iron and a copper ion of this oxidase transfer electrons to O₂, as the last acceptor, to form water.
Each transporter "downstream" is more electronegative than its neighbor "upstream"; oxygen is located in the inferior part of the chain. Thus, the electrons fall in an energetic gradient in the electron chain transport to a more stable localization in the electronegative oxygen atom.