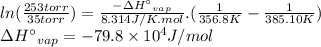Answer:
-79.8 × 10⁴ J/mol
Step-by-step explanation:
Arsine, AsH₃, is a highly toxic compound used in the electronics industry for the production of semiconductors. Its vapor pressure is 35 Torr at 111.95 °C and 253 Torr at 83.6 °C.
Then,
P₁ = 35 torr
T₁ = 111.95 + 273.15 = 385.10 K
P₂ = 253 torr
T₂ = 83.6 + 273.15 = 356.8 K
We can calculate the standard enthalpy of vaporization (ΔH°vap) using the two-point Clausius-Clapeyron equation.
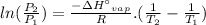
where,
R is the ideal gas constant