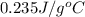Answer : The specific heat of substance is
Explanation :
Formula used :
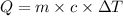
or,
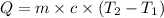
where,
Q = heat needed = 4.90 kJ = 4900 J
m = mass of sample = 894.0 g
c = specific heat of substance = ?
 = initial temperature =
= initial temperature =
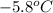
 = final temperature =
= final temperature =
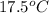
Now put all the given value in the above formula, we get:
![4900J=894.0g* c* [17.5-(-5.8)]^oC](https://img.qammunity.org/2020/formulas/chemistry/college/sntf544s5743kuohn2laqulce3mf4ec3yb.png)
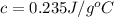
Therefore, the specific heat of substance is