Answer:
1844 years
Step-by-step explanation:
¹⁴C follows a first-order beta-decay according to the following equation.
¹⁴C ⇒ ¹⁴N + β⁻
We can calculate the concentration of ¹⁴C after some time using the following expression.
![ln(([C]_(t))/([C]_(0)) )=-k.t](https://img.qammunity.org/2020/formulas/chemistry/college/gl2hbabcppnjk3yj9t6j1gojwhijw5nbe9.png)
where,
[C]t is the concentration of ¹⁴C after some time
[C]₀ is the original concentration of ¹⁴C
k is the rate constant
t is the time elapsed
We can calculate the rate constant if we know the half-life (t1/2) using the following expression.
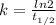
Half-life of ¹⁴C is 5730 years. Then,
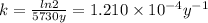
The elapsed time when the concentration of ¹⁴C is 80% of original is:
![ln((0.8[C]_(0))/([C]_(0)) )=-1.210* 10^(-4) y^(-1) * t\\t = 1844y](https://img.qammunity.org/2020/formulas/chemistry/college/dybpe29lrqwgcxntqwhr7meffs6aj8g8pz.png)