Answer: The molarity of KOH solution is 0.0663 M.
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of KHP = 0.4877 g
Molar mass of KHP = 204.22 g/mol
Putting values in equation 1, we get:
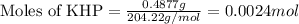
The chemical reaction for the formation of chromium oxide follows the equation:
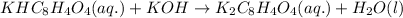
By Stoichiometry of the reaction:
1 mole of KHP reacts with 1 mole of KOH.
So, 0.0024 moles of KHP will react with =
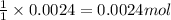 of KOH.
of KOH.
To calculate the molarity of KOH, we use the equation:
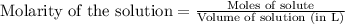
We are given:
Moles of KOH = 0.0024 moles
Volume of solution = 36.21 mL = 0.03621 L (Assuming) (Conversion factor: 1L = 1000 mL)
Putting values in above equation, we get:
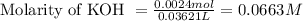
Hence, the molarity of KOH solution is 0.0663 M.