Answer:
0.5 M
Step-by-step explanation:
We have to start with the reaction between NaOH and CH3COOH:
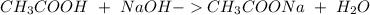
We will have a 1:1 ratio between the acid and the base. The next step then would be the calculation of the moles of NaOH and his convertion to moles of CH3COOH.
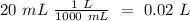
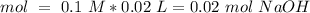
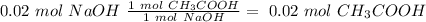
The final step is the calculation of the concentration of the acid.
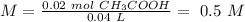
Due to the Ka value we can use the acetic acid as a strong acid.