Answer:
The amount of heat absorbed is 5.183889 kJ .
Step-by-step explanation:
In conversion of water to ice it rejects some heat while in conversion of ice to water it absorbs heat which is called latent heat which is given as 6.02 kJ/mol.
The amount of ice given is 15.5 g.
Converting it to moles as the latent heat is given in per moles:
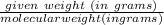
Molecular mass of Hydrogen (H) and Oxygen (O) is 1 u and 16 u respectively.
Molecular mass of water is 18 g (
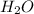 ⇒ 2*1+16=18 ).
⇒ 2*1+16=18 ).
mole = 15.5/18 ≈ 0.8611 moles
Therefore the amount of heat absorbed by 15.5 g of ice ( 0.8611 moles) = Latent heat * moles
Heat absorbed = 6.02*0.8611
= 6.02*(15.5/18)
≈ 5.183889 kJ