Answer:
The pressure increases by 10% of the original pressure
Thus the new pressure is 1.1 times the original pressure.
Step-by-step explanation:
We are given;
- Initial temperature as 30°C, but K = °C + 273.15
- Thus, Initial temperature, T1 =303.15 K
- Final temperature, T2 is 333.15 K
We are required to state what happens to the pressure;
- We are going to base our arguments to Pressure law;
- According to pressure law, the pressure of a gas and its temperature are directly proportional at a constant volume
- That is; P α T
- Therefore, at varying pressure and temperature
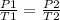
Assuming the initial pressure, P1 is P
Rearranging the formula;
[tex]P2=\frac{P1T2}{T1}[/tex]
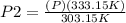
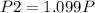
= 1.10 P
The new pressure becomes 1.10P
This means the pressure has increased by 10%
We can conclude that, the new pressure will be 1.1 times the original pressure.